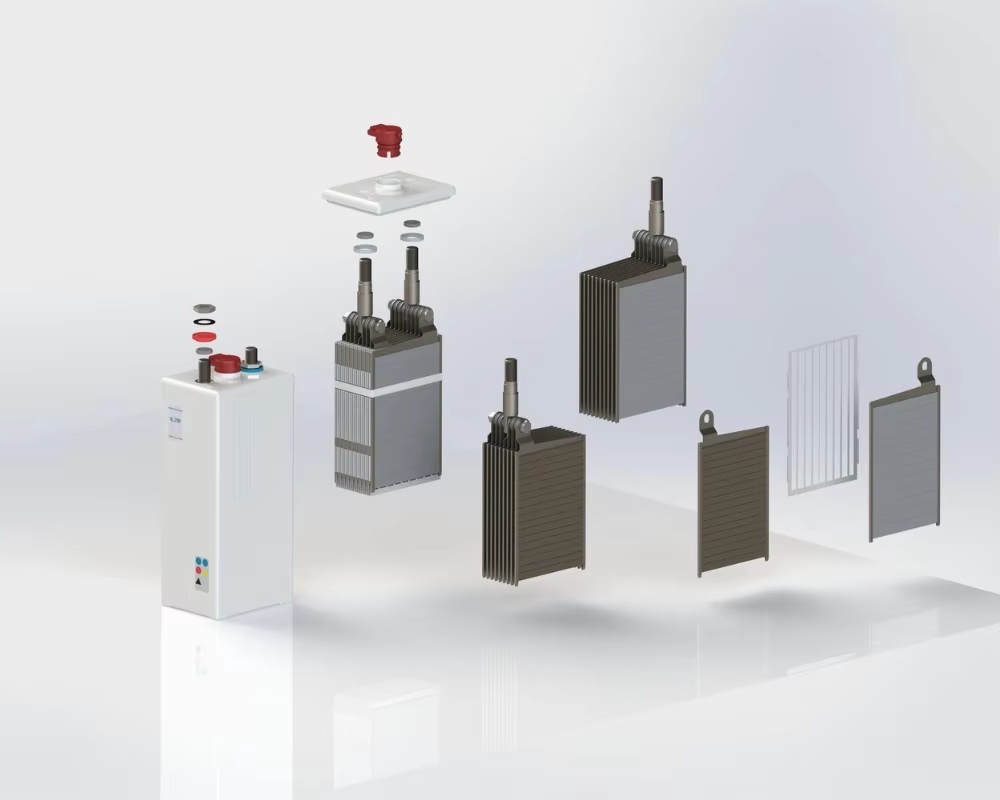
Nickel-Cadmium (Ni-Cd) batteries are a type of rechargeable battery that have been used for decades in industrial and commercial applications. Known for their durability and resilience, Ni-Cd batteries operate using nickel oxide hydroxide as the positive electrode and metallic cadmium as the negative electrode, with a potassium hydroxide electrolyte.
The electrochemical reaction during discharge involves the transfer of hydroxyl ions, which generates electricity. These batteries typically offer a nominal cell voltage of 1.2V and are available in both cylindrical and prismatic forms. Although their energy density is lower than that of lithium-ion batteries, Ni-Cd cells excel in environments with extreme temperatures and frequent cycling.
Applications where reliability and endurance are critical—such as emergency lighting, aircraft systems, and railway signaling—often rely on Ni-Cd technology. Their long service life and ability to deliver full-rated capacity in high-drain scenarios make them a dependable choice.