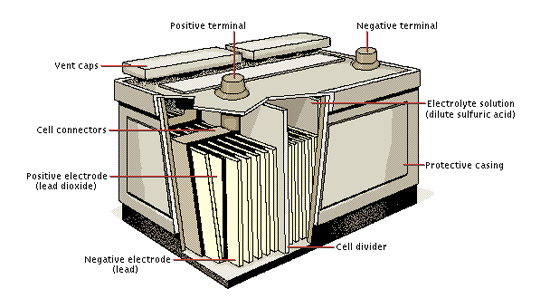
Lead-acid batteries are a type of rechargeable battery widely used for various applications, including automotive starting batteries and backup power systems.
- Basic Structure:
- A lead-acid battery consists of a plastic container housing a series of lead dioxide (PbO2) positive plates and sponge lead (Pb) negative plates. These plates are immersed in a solution of sulfuric acid (H2SO4), known as the electrolyte.
- Chemical Reactions During Discharge:
- When the battery is in use (discharge phase), a chemical reaction takes place between the lead dioxide (positive plate) and the sponge lead (negative plate) in the presence of sulfuric acid.
- The chemical reaction at the positive plate (cathode) is: PbO2 + SO4^2- + 4H^+ + 2e^- → PbSO4 + 2H2O
- The chemical reaction at the negative plate (anode) is: Pb + SO4^2- → PbSO4 + 2e^-
- Overall, the reaction during discharge results in the conversion of lead dioxide and sponge lead into lead sulfate and the release of electrical energy.
- Electron Flow:
- Electrons are released during the chemical reactions at the negative plate and flow through an external circuit, providing electrical power for connected devices.
- Sulfuric Acid Depletion:
- As the battery discharges, the sulfuric acid in the electrolyte combines with the lead plates, leading to the formation of lead sulfate on both positive and negative plates.
- The concentration of sulfuric acid in the electrolyte decreases during discharge.
- Chemical Reactions During Charging:
- When the battery is recharged, a reverse chemical reaction occurs. Applying an external voltage causes lead sulfate to break down, reforming lead dioxide on the positive plate and sponge lead on the negative plate.
- The overall charging reaction is the reverse of the discharge reactions.
- Hydrogen and Oxygen Gas Evolution:
- During charging, hydrogen gas (H2) is evolved at the negative plate, and oxygen gas (O2) is evolved at the positive plate. This is known as gassing.
- Water (H2O) is added to the battery periodically to compensate for the water lost during gassing.
- Cycle Life:
- Lead-acid batteries have a finite number of charge-discharge cycles. Over time, the repeated chemical reactions can lead to the accumulation of lead sulfate on the plates, reducing the battery’s capacity.
Lead-acid batteries are known for their reliability, low cost, and ability to deliver high current, making them suitable for applications such as automotive starting, uninterruptible power supplies (UPS), and backup power systems. However, they require proper maintenance, including checking and replenishing the electrolyte and ensuring proper charging.