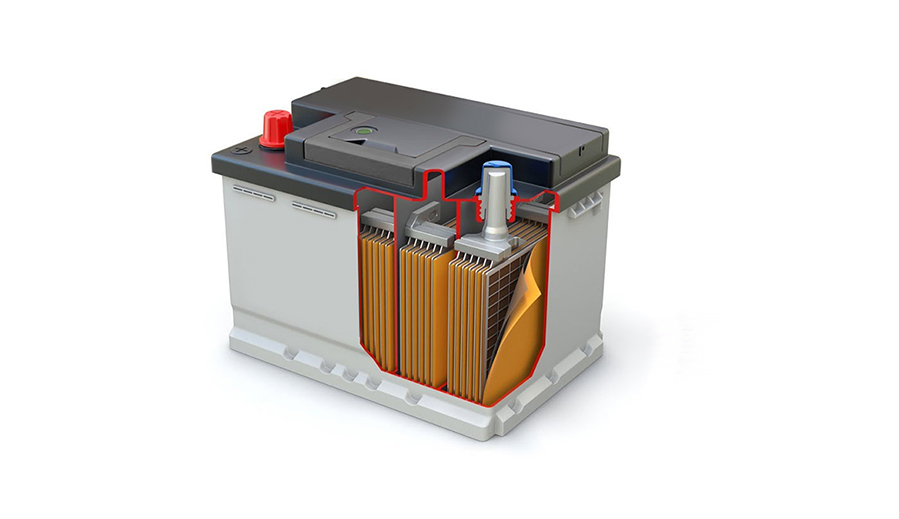
Lead-acid batteries consist of positive and negative plates immersed in an electrolyte. These plates are the primary components responsible for the electrochemical reactions that occur during the charging and discharging cycles of the battery. The two types of plates are the positive plate and the negative plate.
- Positive Plate:
- The positive plate is typically made of lead dioxide (PbO2). Lead dioxide is a lead compound in a crystalline form. The grid structure of the positive plate is often made of a lead-antimony alloy, although lead-calcium alloys are used in some applications for reduced water loss. The lead dioxide serves as the active material during the discharge phase of the battery.
- Negative Plate:
- The negative plate is usually made of sponge lead (Pb). The grid structure for the negative plate is also commonly composed of a lead-antimony alloy, though lead-calcium alloys can be used. The sponge lead acts as the active material during the discharge phase of the battery.
During the discharge cycle of the lead-acid battery, a chemical reaction occurs at both the positive and negative plates:
- At the Positive Plate (during discharge):
- Lead dioxide (PbO2) undergoes a chemical reaction and releases oxygen ions.
- PbO2 + 4H+ + SO4^2- + 2e^- → PbSO4 + 2H2O
- At the Negative Plate (during discharge):
- Sponge lead (Pb) reacts with sulfuric acid (H2SO4) to produce lead sulfate and release electrons.
- Pb + SO4^2- → PbSO4 + 2e^- + 2H+
These reactions result in the production of lead sulfate at both the positive and negative plates. During the charging cycle, when an external voltage is applied to the battery, the reactions are reversed, and lead sulfate is converted back into lead dioxide at the positive plate and sponge lead at the negative plate.
The electrolyte, which is a solution of sulfuric acid (H2SO4) in water, facilitates the movement of ions between the positive and negative plates during the electrochemical reactions, allowing for the flow of electric current and the storage of energy in the battery.