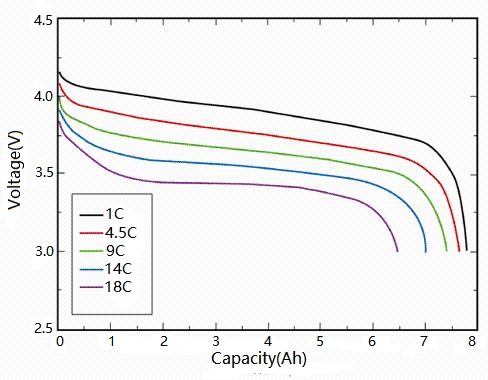
The self-discharge rate of lead-acid batteries is influenced by several factors.
- Temperature: Higher temperatures accelerate the chemical reactions within the battery, leading to a higher self-discharge rate. Conversely, lower temperatures slow down these reactions, reducing the self-discharge rate. However, extreme cold can also affect the battery’s capacity and performance.
- State of Charge (SOC): The self-discharge rate is typically higher when the battery is at a higher state of charge. For example, a fully charged battery will self-discharge more rapidly than a partially charged one.
- Battery Age and Condition: As batteries age, their internal resistance increases, which can lead to a higher self-discharge rate. Additionally, batteries that have been subjected to repeated deep discharges or overcharging may have a higher self-discharge rate.
- Battery Chemistry: Different types of lead-acid batteries have different self-discharge rates. For example, valve-regulated lead-acid (VRLA) batteries, such as AGM and gel batteries, typically have lower self-discharge rates than flooded lead-acid batteries.
- Battery Design: The design of the battery, including the type of electrodes and separators used, can affect the self-discharge rate.
- Impurities: Impurities in the battery’s electrolyte can lead to increased self-discharge rates.
- Parasitic Loads: Parasitic loads, such as small electronic devices connected to the battery, can drain the battery and increase the self-discharge rate.
- Storage Conditions: Proper storage conditions, such as a cool, dry environment, can help reduce the self-discharge rate.
By considering these factors, battery users can take steps to minimize the self-discharge rate and maximize the battery’s lifespan.