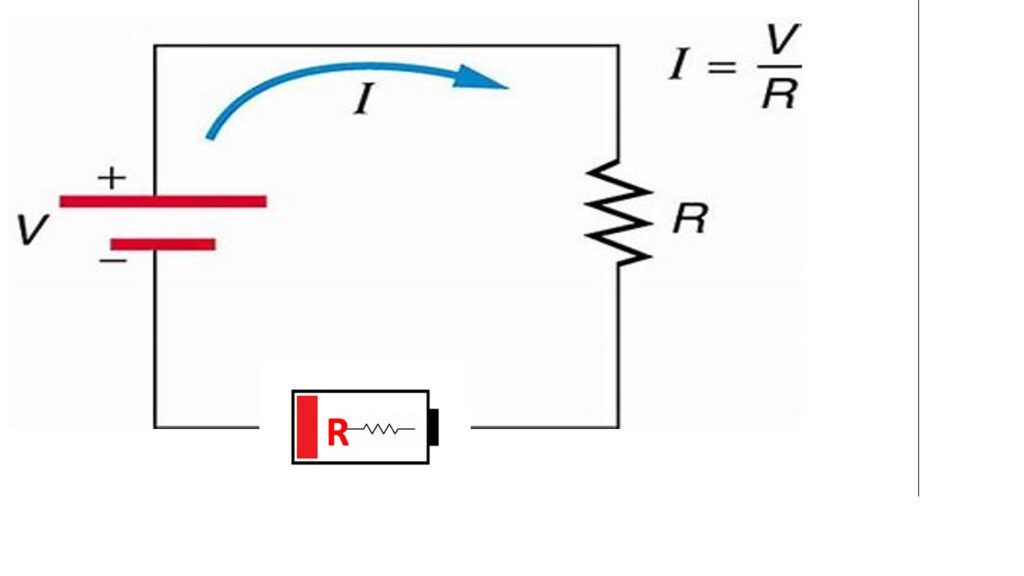
The internal resistance of lead-acid batteries tends to increase over time due to several reasons.
- Sulfation: Lead-acid batteries can experience sulfation, where lead sulfate crystals form on the battery plates during discharge. Over time, these crystals can become hardened and difficult to remove during charging, leading to increased internal resistance.
- Plate degradation: The active material on the battery plates can degrade over time due to repeated charge-discharge cycles, exposure to high temperatures, or improper maintenance. This degradation can result in reduced surface area for chemical reactions to occur, leading to higher internal resistance.
- Corrosion: Corrosion of the battery terminals and internal connections can increase resistance within the battery, hindering the flow of electrical current.
- Electrolyte loss: Evaporation or leakage of electrolyte from the battery can alter the concentration of sulfuric acid, affecting its conductivity and increasing internal resistance.
- Temperature effects: Extreme temperatures can affect the physical properties of the battery materials, leading to changes in internal resistance. High temperatures can accelerate chemical reactions and material degradation, while cold temperatures can slow down reactions and increase viscosity, both of which can contribute to increased resistance.
- Age: As lead-acid batteries age, their internal components can degrade and accumulate contaminants, leading to increased resistance over time.
Overall, these factors contribute to the gradual increase in internal resistance of lead-acid batteries, which can result in reduced performance and capacity over their lifespan. Proper maintenance, including regular charging and periodic inspection, can help mitigate some of these issues and prolong the battery’s useful life.